Coronavirus: How We Got Here
Text by Richard Kessin
Photos Courtesy of the CDC
The March 12 issue of Nature has two dense scientific articles on the discovery of SARS-CoV-2. Though most of the data were already available, the narrative, all in one place, is gripping. The first article describes a 41-year-old man who was seen in The Central Hospital of Wuhan on Dec. 26, 2019. He had been sick for six days and reported fever, chest tightness, unproductive cough, pain and weakness. He did not have influenza. He needed help breathing and had abnormal lungs on chest x-rays and CAT scans. About 12 days after the appearance of symptoms, he was transferred to intensive care.
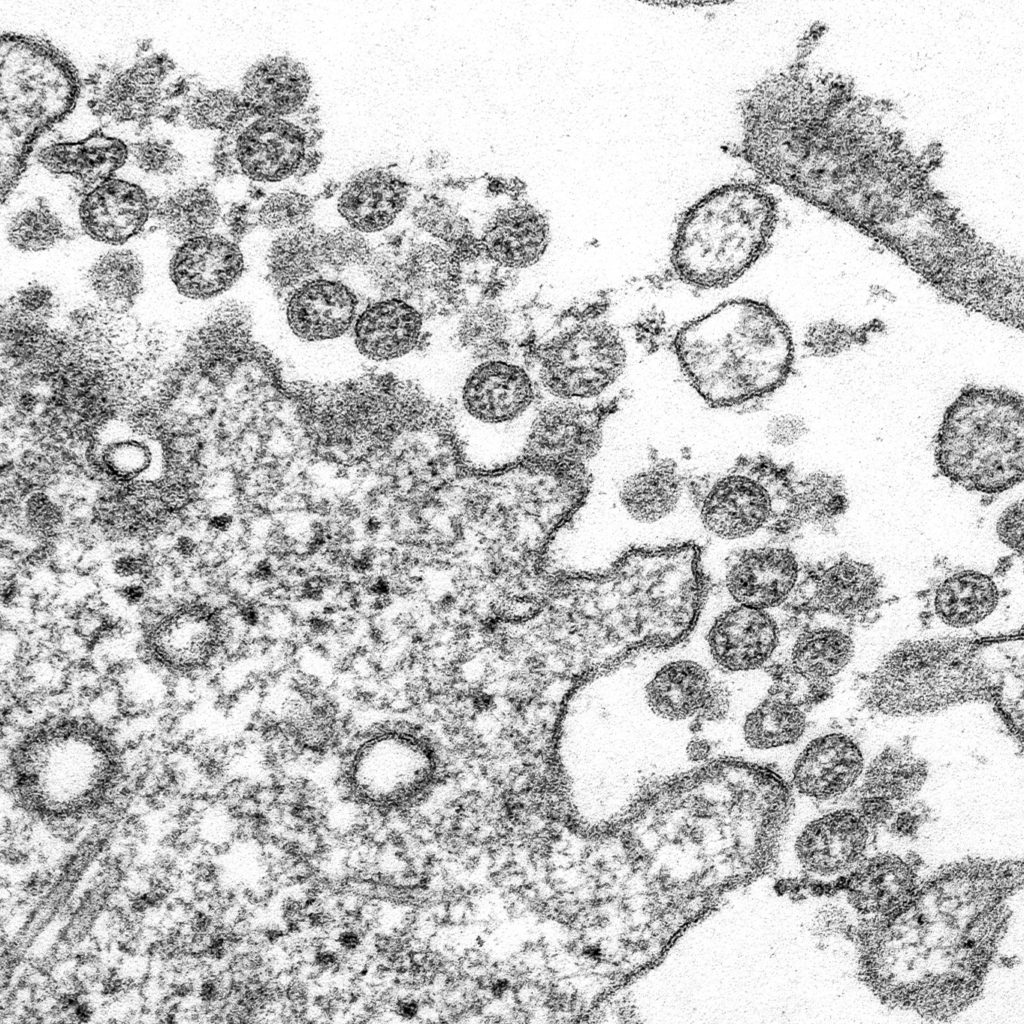
His physicians did a bronchoalveolar lavage—they put saline deep into one lung and recovered 200 microliters of wash fluid— about four drops. The researchers hoped to find a virus, and they did—about 100 million particles. Virologists and biologists are used to large numbers, but 100 million in 0.2 ml is a lot of virus. The paper did not say whether the patient survived.
The team sequenced the genome of the virus and made it available to the world in mid-January. Virus genomes are made of four nucleotide subunits: A, C, G, and U. In a certain order they code for the proteins that make up the virus. When the scientists in Wuhan sequenced the RNA in their sample, they found one type of virus with almost 30,000 nucleotide subunits, and from that sequence they learned that they had a new and very dangerous coronavirus. They also knew that the virus had made its way into a local indoor fish and seafood market where their patient had worked. In extensive computer comparisons—a kind of 23 and Me for viruses—they learned that the new virus is most closely related to a bat virus that had been found in a cave. The market also sold live hedgehogs, badgers, snakes and doves, and the new coronavirus may have infected one of these species before it jumped to humans.
By the middle of January, the novel disease had started to spread rapidly, eventually affecting more that 100,000 people and killing thousands. Hospitals were overwhelmed, despite the efforts of the authorities to create new hospitals and declaring a stay-at-home policy to break the chain of infection. At first there were not enough supplies, nurses or physicians to treat all patients. That is the situation that the United States and other countries may soon be in.
Epidemics have been studied for a long time and mathematics has made its contribution with a factor called R0 (pronounced R naught). It describes the number of people a single patient infects. With measles, R0 is very high, 12 to 18. Think Disneyland. SARS-Cov-2 is less infectious than the measles virus, and its R0 is estimated to be 2-3. R0 for influenza is about 1.3. An R0 value above one means that an epidemic will expand. Reducing R0 to one lets the infection smolder in the population. Reducing it to less than one drives the virus to extinction, as happened with the first SARS virus in 2003. That is the idea behind sheltering in place, hand washing, and personal protective gear.
In the medium term, there are better prospects. Most RNA viruses need a unique enzyme to copy their genomes and make new viruses. They also make a few large proteins and then chop them up to make smaller proteins. Two unique enzymes carry out these steps: an RNA polymerase and a protease, neither of which is present in uninfected human cells. Inhibitors of the enzymes in HIV and hepatitis C, which are also RNA-based viruses, have essentially cured these diseases. A recent report in the New England Journal of Medicine tested two inhibitors that work against HIV against SARS-CoV-2 but these failed to stop it. There are many other inhibitors to try.
Recent reports from China and France suggest that a combination of chloroquine, an antimalarial drug, and azithromycin, an antibiotic, stops coronavirus reproduction. These reports described small tests and the combination may yet work. These drugs should not be promoted as an answer to the epidemic. For the moment, these results are too thin a reed to grasp.
The ultimate test is a vaccine, and a number have entered phase-one trials, where they are tested for safety, dose, and the immune responses they provoke. They usually present humans with a coronavirus spike protein, whose role in to bind to human cells. It will take at least a year to complete phases two and three. There will be pressure to speed the process up. That involves risk, but it may be inevitable.
Richard Kessin is professor emeritus of pathology and cell biology at the Vagelos College of Physicians and Surgeons of Columbia University. Email: Richard.Kessin@gmail.com,